silver nitrate reacts with potassium chloride to produce solid silver chloride and what substance
What is Silver Nitrate?
Silver nitrate is a chemical compound with the formula AgNOiii. It consists of an ionic bail between the argent cation (Ag+) and the nitrate anion (NOthree –). Due to the ionic nature of this compound, it readily dissolves in water and dissociates into its constituent ions.
Silvery nitrate is a precursor to many compounds of silverish, including the silverish compounds used in photography. When compared to silver halides, which are used in photography due to their sensitivity to light, AgNO3 is quite stable when exposed to light.
Structure of AgNO3
An analogy describing the structure of the silver nitrate molecule is provided beneath. It tin be observed that silver has an oxidation number of ane in this compound.
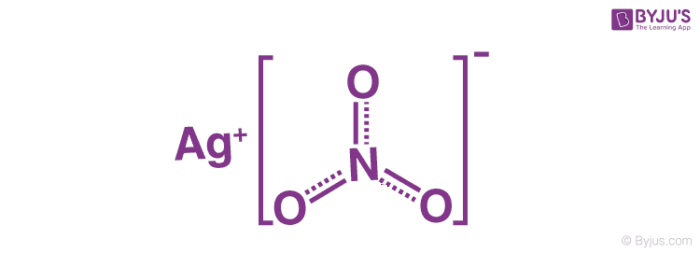
The nitrate ion described above consists of one nitrogen cantlet which is surrounded by 3 oxygen atoms. The nitrogen-oxygen bonds in this ion are similar to each other. The formal charge assigned to the nitrogen atom is -1, whereas each oxygen atom holds a accuse of -⅔. The cyberspace accuse associated past the nitrate ion is -1, which is quenched past the +1 charge held by the Ag+ ion via an ionic bond in AgNO3. It can be noted that the construction of the nitrate ion is stabilized by resonance.
Backdrop of Silvery Nitrate
Some important physical and chemic backdrop of silver nitrate are listed in this subsection.
Physical Properties
- The molar mass of silver nitrate is 169.872 grams per mole.
- AgNOthree equally a colourless appearance in its solid land and is odourless.
- In its solid state, it has a density of 4.35 grams per cubic centimetre. Its density in the liquid land at a temperature of 210oC corresponds to three.97 g/cmthree.
- The melting and boiling points of argent nitrate are 482.8 Chiliad and 713 K respectively. Even so, this compound tends to decompose at temperatures budgeted its boiling point.
- Silverish nitrate, like most ionic compounds, dissolves readily in water. Its solubility in water corresponds to 122g/100mL at 0oC and 256g/100mL at a temperature of 25o
- The crystal structure of AgNO3 is orthorhombic.
Chemical Properties
- The hazards of AgNO3 include its toxic and corrosive nature.
- The reaction between silver nitrate and ethanol is explosive.
- The silver present in this chemical compound is displaced past copper, which forms copper nitrate. The chemical equation for this reaction is given past 2AgNO3 + Cu → Cu(NO3)ii + 2Ag
- When heated to 440oC, this chemical compound completely decomposes to requite oxygen, nitrogen dioxide, and silver.
Information technology tin be noted that even though metallic nitrates generally decompose to yield metal oxides, the decomposition reaction of silverish nitrate gives rise to elemental silver because silvery oxide decomposes at an even lower temperature than AgNO3.
Uses of Argent Nitrate
Silver nitrate has a wide range of applications in many fields such as biology, chemical synthesis, and medicine. Some of these uses of AgNO3 are listed below.
- Silvery nitrate is a very versatile chemical compound considering the nitrate ion can be replaced past other ligands that tin can bind to the silverish ion.
- Due to the ability of this compound to class a precipitate of silver halides when treated with halide ions, it is used while making photographic films.
- Many silverish-based explosives tin be prepared with a precipitation reaction of silvery nitrate.
- In the field of inorganic chemical science, halides are extracted with the help of this compound.
- The branch of chemical science known as analytical chemistry uses this reaction to cheque for the presence of halide anions such as the iodide, bromide, or chloride ions.
- Mixtures of alkenes can exist separated with the help of this compound since the silver cation binds with alkenes in a reversible way.
- When diluted with water to a concentration of 0.five%, argent nitrate can serve equally an antiseptic in many medical setups.
- A diluted solution of AgNO3 can exist administered to the eyes of a baby which is born to a mother suffering from gonorrhea, which combats the gonococcal bacteria and protects the baby from the onset of blindness.
- This compound is also known to be used for the treatment and the removal of unwanted warts in human beings.
Ofttimes Asked Questions
What are the uses of silvery nitrate?
Argent nitrate is widely used in many organic synthesis reactions in several ways. For example, for the deprotection and oxidation reactions. The Ag+ ion reversibly binds alkenes, and selectively adsorbing silver nitrate tin can exist used to isolate alkene mixtures. The resulting adduct tin can be decomposed (in order to release the costless alkene) with ammonia. Argent nitrate has been, in the past, used for silver staining (a procedure that employs silver or silver compounds to selectively alter the appearance of a specific object). This chemical compound is also used in medicine owing to its clarified qualities.
Is silver nitrate dangerous?
Silver nitrate is an oxidant and must, therefore, exist kept away from organic compounds. Despite its widespread usage (especially in extremely low amounts) for the prevention of gonorrhea, and to stop bleeding from the olfactory organ, silver nitrate is often highly toxic and corrosive. Short-term exposure to this compound does not cause whatever immediate side effects apart from the development of a violet, dark-brown or blackness stain on the part of the skin that was in contact with the silver nitrate. However, exposure to this compound over long periods of fourth dimension is often accompanied by damage to the optics. This compound is widely classified as an irritant to the skin and the optics.
How is silver nitrate prepared?
Silvery nitrate is usually prepared by combining silverish with nitric acid. Mutual silver objects used in these reactions include silver bullions and silver foils. The products formed in this reaction include silver nitrate, water, and nitrogen oxides. The by products of this chemical reaction depend on the nitric acid concentration that is used. It is important to note that this reaction must be carried out nether a fume hood because of the evolution of poisonous oxides of nitrogen during the chemical reaction.
Thus, the important physical and chemical backdrop of silver nitrate are briefly described along with its backdrop. To learn more about this compound and other silver compounds such every bit silver chloride, register with BYJU'South and download the mobile application on your smartphone.
Source: https://byjus.com/chemistry/silver-nitrate/
0 Response to "silver nitrate reacts with potassium chloride to produce solid silver chloride and what substance"
ارسال یک نظر